Abstract
Introduction
Axicabtagene ciloleucel (axi-cel) and tisagenlecleucel (tisa-cel), CD19 directed CAR T cells, are effective treatments (tx) for relapsed/refractory (R/R) aggressive B-cell lymphomas (B-NHL). We present a multicenter retrospective study of safety, patterns of use, resource utilization, and efficacy with commercial axi-cel and tisa-cel in 7 US centers that offer both products for R/R B-NHL.
Methods
Data collection included all patients (pts) who underwent apheresis from 5/1/2018 (when centers had a choice of either axi-cel or tisa-cel) through 7/31/2019, with a data cutoff of 12/31/20. Pt and tx characteristics are summarized descriptively. Cytokine release syndrome (CRS) and neurologic toxicity (NT) were graded per ASTCT consensus. Pt selection, toxicity management, and response assessment followed institutional practices. Univariable and multivariable analyses (MVA) with forward selection were performed to determine the association between baseline/tx characteristics, and response and toxicity.
Results
251 pts underwent apheresis, 161 (64%) for axi-cel, and 90 (36%) for tisa-cel. Twelve (7%) axi-cel and 8 (9%) tisa-cel pts died from lymphoma progression prior to infusion.
Among infused pts, the median age at apheresis was 59 years (range: 18-86) for axi-cel pts and 67 years (range: 37-89) for tisa-cel pts, with 35% of axi-cel and 61% of tisa-cel pts aged ≥ 65 years. By histology, 81% of axi-cel pts had either DLBCL or HGBL, compared to 91% of tisa-cel pts. Median number of prior therapies was 3 (range: 2-10) for axi-cel and 4 (range: 2-7) for tisa-cel pts, with 72% of axi-cel and 85% of tisa-cel pts receiving ≥ 3 prior therapies. Forty percent of axi-cel and 21% of tisa-cel pts had primary refractory disease. Bridging therapy was given in 63% of axi-cel and 73% of tisa-cel pts. Median time from apheresis to infusion was 28 days for axi-cel and 44 days for tisa-cel. Sixty-one percent of axi-cel and 44% of tisa-cel pts would have been ineligible for ZUMA-1 and JULIET, respectively.
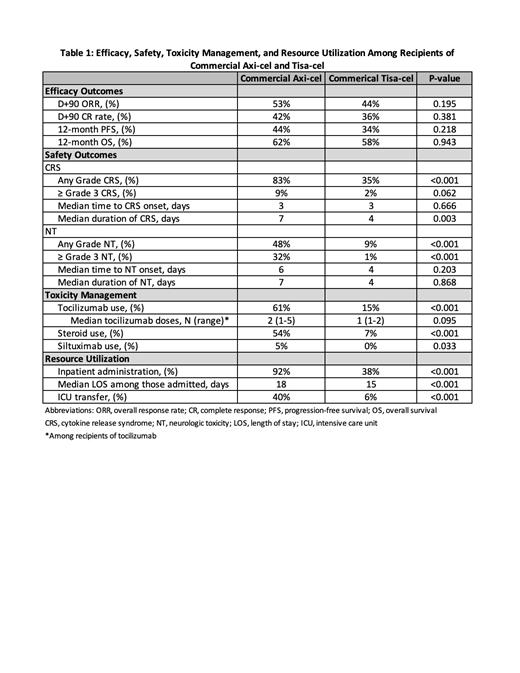
CRS occurred in 123 (83%) axi-cel pts; Grade ≥3 CRS in 13 (9%) (Table 1). CRS occurred in 29 (35%) tisa-cel pts; Grade ≥3 CRS in 2 (2%). NT occurred in 72 (48%) axi-cel recipients; Grade ≥3 NT in 47 (32%). NT occurred in 7 (9%) tisa-cel recipients; Grade ≥3 NT in 1 (1%). Median onset of CRS and NT was 3 and 6 days, respectively, with axi-cel, and 3 and 4 days, respectively, with tisa-cel. Tocilizumab was administered in 61% of axi-cel pts with 54% receiving steroids. In tisa-cel pts, tocilizumab was administered in 15% of cases, with 7% receiving steroids. Inpatient CAR T-cell infusion occurred in 92% of axi-cel and 38% of tisa-cel pts. The median hospital length of stay (LOS) was 18 days for axi-cel pts and 15 days in tisa-cel pts. Intensive care unit (ICU) transfer occurred in 40% of axi-cel pts and 6% of tisa-cel pts.
Response for infused pts was assessed at day 90 or at time of clinical progression. Of 142 axi-cel pts evaluable at day 90, the ORR was 53% with 42% achieving a CR. Of 81 tisa-cel pts evaluable at day 90, the ORR was 44% with 36% achieving a CR. With a median follow-up of 12.2 months in axi-cel recipients, the 12-month PFS was 44% with 12-month OS of 62%. With median follow-up of 13 months in tisa-cel recipients, the 12-month PFS was 34% with 12-month OS of 58%.
On MVA, peak ferritin ≥ 5000 ng/ml was associated with worse PFS (HR 2.19; 95% CI, 1.34-3.59; P=0.002) and OS (HR 3.44; 95% CI, 1.98-5.97; P<0.001). Tx with tisa-cel was associated with a lower incidence of ≥ Grade 3 NT (OR 0.03; 95% CI, 0.01-0.25; P=0.001), while an elevated LDH prior to lymphodepleting chemotherapy (OR 3.75; 95% CI, 1.35-10.43; P=0.011) and an ECOG PS >1 (OR 5.83; 95% CI, 1.12-30.45; P=0.037) were associated with severe NT.
Conclusions
In this retrospective multicenter analysis by centers with prescription choice of either axi-cel or tisa-cel for R/R B-NHL, baseline characteristics differed between recipients of axi-cel and tisa-cel. Tisa-cel recipients were more likely to be older and heavily pretreated, and less likely to have primary refractory disease. In an unmatched comparison, ORR, PFS, and OS appeared comparable between axi-cel and tisa-cel recipients. Safety appeared more favorable than reported in the pivotal trials. In comparison to axi-cel, tisa-cel was associated with a lower incidence and severity of CRS and NT, lower utilization of tocilizumab and steroids, lower rate of ICU transfer, and shorter median hospital LOS.
Riedell: Xencor: Research Funding; Calibr: Research Funding; Karyopharm: Consultancy, Membership on an entity's Board of Directors or advisory committees; Bayer: Membership on an entity's Board of Directors or advisory committees; Celgene/BMS: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Janssen: Membership on an entity's Board of Directors or advisory committees; Takeda: Consultancy, Membership on an entity's Board of Directors or advisory committees; BeiGene: Consultancy; Novartis: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Kite/Gilead: Research Funding, Speakers Bureau; Tessa Therapeutics: Research Funding; MorphoSys: Research Funding. Nastoupil: Denovo Pharma: Other: DSMC; Janssen: Honoraria, Research Funding; Bayer: Honoraria; Gilead/Kite: Honoraria, Research Funding; Caribou Biosciences: Research Funding; Novartis: Honoraria, Research Funding; Genentech: Honoraria, Research Funding; Pfizer: Honoraria, Research Funding; TG Therapeutics: Honoraria, Research Funding; Epizyme: Honoraria, Research Funding; Takeda: Honoraria, Other: DSMC, Research Funding; Bristol Myers Squibb/Celgene: Honoraria, Research Funding; IGM Biosciences: Research Funding; MorphoSys: Honoraria; ADC Therapeutics: Honoraria. Perales: Novartis: Honoraria, Other; Equilium: Honoraria; Karyopharm: Honoraria; Miltenyi Biotec: Honoraria, Other; Incyte: Honoraria, Other; Merck: Honoraria; Medigene: Honoraria; Kite/Gilead: Honoraria, Other; Cidara: Honoraria; Celgene: Honoraria; Bristol-Myers Squibb: Honoraria; MorphoSys: Honoraria; Nektar Therapeutics: Honoraria, Other; NexImmune: Honoraria; Omeros: Honoraria; Takeda: Honoraria; Servier: Honoraria; Sellas Life Sciences: Honoraria. Maziarz: Athersys: Other: Data and Safety Monitoring Board, Patents & Royalties; Artiva Therapeutics: Consultancy; Allovir: Consultancy, Research Funding; Bristol-Myers, Squibb/Celgene,, Intellia, Kite: Honoraria; CRISPR Therapeutics: Consultancy; Omeros: Research Funding; Intellia: Honoraria; Novartis: Consultancy, Other: Data and Safety Monitoring board, Research Funding; Incyte Corporation: Consultancy, Honoraria; Vor Pharma: Other: Data and Safety Monitoring Board. McGuirk: Allovir: Consultancy, Honoraria, Research Funding; Bellicum Pharmaceuticals: Research Funding; Novartis: Research Funding; EcoR1 Capital: Consultancy; Astelllas Pharma: Research Funding; Fresenius Biotech: Research Funding; Magenta Therapeutics: Consultancy, Honoraria, Research Funding; Kite/ Gilead: Consultancy, Honoraria, Other: travel accommodations, expense, Kite a Gilead company, Research Funding, Speakers Bureau; Gamida Cell: Research Funding; Novartis: Research Funding; Juno Therapeutics: Consultancy, Honoraria, Research Funding; Pluristem Therapeutics: Research Funding. Bachanova: FATE: Membership on an entity's Board of Directors or advisory committees, Research Funding; Incyte: Research Funding; KaryoPharma: Membership on an entity's Board of Directors or advisory committees; Gamida Cell: Membership on an entity's Board of Directors or advisory committees, Research Funding. Schuster: Genentech/Roche: Consultancy, Research Funding; Loxo Oncology: Consultancy; Tessa Theraputics: Consultancy; Pharmaclyclics: Research Funding; Juno Theraputics: Consultancy, Research Funding; BeiGene: Consultancy; Alimera Sciences: Consultancy; Acerta Pharma/AstraZeneca: Consultancy; Abbvie: Consultancy, Research Funding; Novartis: Consultancy, Honoraria, Patents & Royalties, Research Funding; Nordic Nanovector: Consultancy; Celgene: Consultancy, Honoraria, Research Funding; Adaptive Biotechnologies: Research Funding; Merck: Research Funding; Incyte: Research Funding; TG Theraputics: Research Funding; DTRM: Research Funding. Bishop: Arcellx, Autolus, Bristol-Myers Squibb, CRISPR, Kite/Gilead, and Novartis: Consultancy, Research Funding; Bristol-Myers Squibb and Kite/Gilead: Other: fees for non-CME/CE services . Porter: Novartis: Membership on an entity's Board of Directors or advisory committees, Patents & Royalties, Research Funding; National Marrow Donor Program: Membership on an entity's Board of Directors or advisory committees; Janssen: Membership on an entity's Board of Directors or advisory committees; Kite/Gilead: Membership on an entity's Board of Directors or advisory committees; Unity: Patents & Royalties; Wiley and Sons Publishing: Honoraria; Incyte: Membership on an entity's Board of Directors or advisory committees; GenenTech: Current Employment, Current equity holder in publicly-traded company; DeCart: Membership on an entity's Board of Directors or advisory committees; ASH: Membership on an entity's Board of Directors or advisory committees; American Society for Transplantation and Cellular Therapy: Honoraria.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal